Quality Creates Value
Pharmaceutical companies play a critical role in global health. While much of their time is spent on innovation, their ability to deliver essential medicines with quality, consistency, dependability, affordability, and in sufficient volumes is essential to the wellbeing of every community. Given the complexity of pharmaceutical manufacturing and the high stakes involved, the importance of quality in drug development and operations of these companies cannot be overstated. Although quality may often seem like an abstract or vague concept, its absence can have severe consequences, such as compromised patient safety, product recalls, license suspension or revocation, warning letters, consent decrees, and fines and penalties. Such failures can lead to a reputational disaster for organizations operating in this highly trust-sensitive market.
The notion of quality and good manufacturing practices (GMP) reflects the original quality philosophy that originated in the 1930s (US Food and Drug Administration, 2023). While this approach generally served the pharmaceutical industry well, current trends in quality organizations and shifting market demands necessitate that companies adopt a more forward-thinking and holistic approach to ensure quality remains a top priority.
Pharmaceutical quality in the spotlight
The pharmaceutical industry constantly experiences growth in size and complexity, making it essential to have a sophisticated system in place to ensure that patients receive high-quality products. Facing regulatory scrutiny, increased public attention, and the growing power of well-informed patients, pharmaceutical companies are prioritizing patient safety and product quality more than ever. Quality teams serve as the backbone of this priority, ensuring that products meet the highest standards of quality, safety, and efficacy.
As Dr. Lothar Halmer, Chief Quality Officer at Boehringer Ingelheim, recently stated: “The trust of our patients is our greatest resource … It takes a lot of time to build trust, but very little to destroy it.” (Porsche Consulting The Magazine, 2022). Understanding and building trust is a critical imperative for pharmaceutical organizations. In our previous research, “Trust: a value imperative for pharma” (WittKieffer, 2023), we introduced the hierarchy of trust, a framework designed to demystify the often elusive and challenging-to-measure concept of trust in the pharmaceutical industry. The foundational layer of the hierarchy of trust consists of “benefits of medicines,” which encompasses not only innovation and efficacy but also safety and quality — the building blocks of patient and public trust.
Hierarchy of Trust
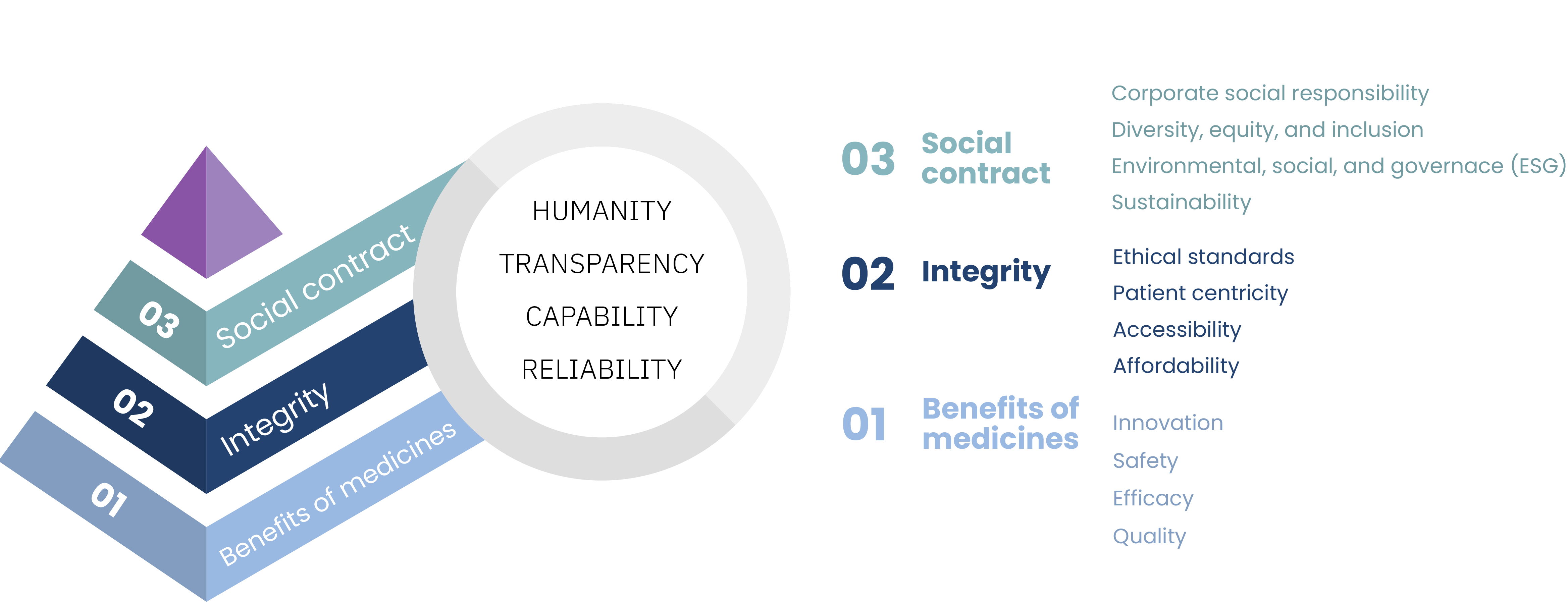
With this background in mind, here are the top 10 considerations impacting the evolution of the quality function in the pharmaceutical industry today.
Supply chain resilience. The Covid-19 pandemic, geopolitical events, and economic instability have made supply chains increasingly fragile and complex. Quality functions must adapt to develop and maintain robust risk management systems to identify, assess, and mitigate potential disruptions that could impact product quality, patient safety, and regulatory compliance. By proactively managing risks, quality teams can help ensure a steady supply of active pharmaceutical ingredients (APIs), raw materials, and high-quality products, even in the face of unexpected disruptions.
Increased complexity. The pharmaceutical industry is experiencing a surge in complexity due to the rise of advanced technologies, such as gene and cell therapies, coupled with increasingly globalized operations. This evolution requires new analytical tools and real-time release procedures to effectively manage the unique challenges posed by these advanced therapies. This can lead to a higher risk of inconsistencies, security vulnerabilities, and difficulties in tracking, all of which can have serious consequences for both patients and the pharmaceutical company. Quality functions must rise to meet this complexity by developing rigorous risk management protocols, mitigating the risk of errors, and ensuring strict adherence to regulatory guidelines, ultimately ensuring the delivery of safe and effective products to patients.
Personalization of medicine. The growing trend in patient-centric and technologically advanced solutions is reshaping the future of pharmaceuticals. Key examples include the demand for personalized medicines, holistic and hyper-responsive healthcare, real-world data, and artificial intelligence (AI) applications to analyze this data and derive meaningful insights. This movement towards more sophisticated and tailored approaches in pharmaceuticals holds the potential to revolutionize healthcare and improve patient outcomes. As such, quality teams have to adjust to these changes and ensure that products meet the highest standards in this evolving landscape.
Regulatory scrutiny. Pharmaceutical companies are under pressure to meet the standards of stringent regulatory scrutiny and stay on top of an increasing number of regulatory requirements. With regulatory bodies worldwide implementing stricter quality standards and guidelines, quality functions must stay current with ongoing changes and implement robust compliance programs. This means conducting regular audits, establishing comprehensive training programs, and fostering open lines of communication with regulatory bodies. By staying proactive and vigilant in their compliance efforts, quality teams ensure medicines meet the highest quality and safety standards, and that the company maintains its reputation and market position.
Digital transformation. The pharmaceutical industry is undergoing digital transformation, adopting digital tools and technologies such as data analytics, AI, and automation. Integrating data and technology into holistic quality operations offers numerous advantages, such as delivering real-time insights into process quality and performance, predicting potential quality lapses, enabling rapid decision-making and error reduction, and streamlining compliance with regulatory standards. However, in this highly regulated environment, the quality and integrity of data are crucial. Pharma companies must implement strong computer systems assurance and data integrity practices to maintain regulatory compliance; this involves using validated systems, establishing data governance frameworks, and ensuring data accuracy, completeness, and reliability throughout its entire lifecycle. Quality teams can leverage these technologies to enhance supply chain visibility, traceability, and efficiency, ultimately improving product quality and patient safety.
Expansion of pharmaceutical services. The pharmaceutical industry is witnessing a rise in the complexity of quality organizations, driven by the increasing sophistication of drug development and manufacturing processes, as well as the growing support from third-party suppliers to manage this complexity. The pharmaceutical services market is expanding by more than 8% annually (Houlihan Lokey, 2024), encompassing the entire value chain from research to manufacturing and supply chain to commercialization. Simultaneously, contract development and manufacturing organizations (CDMOs) evolve from mere suppliers to strategic partners, enhancing their technological capabilities, scale, and quality (WittKieffer, 2024). To maintain consistent performance, quality functions at pharmaceutical companies enhance supplier management processes, including rigorous selection, evaluation, and monitoring procedures, such as conducting thorough risk assessments, establishing clear quality agreements, and regularly auditing suppliers to ensure they meet the required standards.
Interconnectivity. The trend towards greater interconnectivity within pharmaceutical companies involves aligning different adjacent functions, such as safety and medical quality, R&D quality, and regulatory affairs, into the global quality function. This alignment can bring several benefits, including improved communication and collaboration between departments, reduced redundancies, and a more holistic approach to quality and regulatory considerations throughout the product development and commercialization process.
Governance. Industry best practice recommends that quality team members be organizationally separated from manufacturing operations to avoid conflicts of interest and ensure the independence and objectivity of quality decisions. This separation, recommended by the FDA (US Food and Drug Administration, 2006), ensures objective decision-making, maintains the integrity of quality oversight, and provides the necessary authority and resources to manage quality risks effectively. The reporting structure in a pharmaceutical company can vary depending on several factors, such as the company’s size, applicable regulatory requirements, governance structures, and the organization’s emphasis on quality.
Quality efficiency. As pharma companies seek to operate with lower costs and shorter timelines, quality functions must strike a balance between cost, speed, and quality, ensuring that cost- and time-saving initiatives do not compromise product quality and patient safety nor hinder innovation. To achieve this balance, quality organizations should implement cost-effective (digital) quality management systems, lean manufacturing principles, or process optimization techniques. Additionally, measuring and monitoring quality performance through key performance indicators and quality metrics help identify trends, highlight areas for improvement, and support data-driven decision-making.
Sustainability. Like most other industries, the pharmaceutical industry, which emits more greenhouse gases than the automotive sector (McMaster University, 2019), is under pressure to reduce its environmental impact. However, there is a dilemma that organizations face when trying to balance sustainability and quality as sustainability initiatives can sometimes compromise the quality of the final product. To overcome this challenge, organizations can adopt a holistic approach that considers both sustainability and quality as strategic imperatives. Quality teams must ensure that any sustainability initiatives implemented do not compromise the quality of the products they produce. Practical examples of quality and sustainability integration in the pharmaceutical industry include eco-friendly packaging, green chemistry, continuous manufacturing, energy management systems, and circular economy practices – and these can lead to improved profitability, too (Pharmaceutical Technology, 2023).
Quality culture as a strategic growth driver
To ensure the quality and safety of their medicines, pharmaceutical organizations are evolving their approaches. Traditionally, they relied on quality control (QC), emphasizing inspection and post-production testing by focusing on “fulfilling quality requirements” (International Organization for Standardization, 2015). Over time, focus shifted towards adopting quality assurance (QA) principles and a proactive approach to quality management. This shift emphasized prevention over detection to provide confidence to both internal and external stakeholders that “quality requirements will be fulfilled” (International Organization for Standardization, 2015).
Despite this progress, a narrow focus on regulatory compliance can hinder pharmaceutical organizations from fully embracing and benefiting from quality and operational excellence. This is where a paradigm shift can occur, and the concept of Quality Culture represents a new frontier in quality management.
Quality Culture differs from traditional approaches in several key ways. First, it addresses the entire organization, not just the quality team. Second, it focuses on underlying mindsets and behaviors, rather than just quality systems and processes. Finally, it considers quality capabilities to be the cornerstone of pan-enterprise vision, allowing quality functions to shift from policing to enabling other business areas (McKinsey, 2022).
At its core, Quality Culture is the mindset and behavior of all employees, and it should be embraced at the very heart of the organization and sponsored from the top. The two building blocks are:
- Leading with quality as a mechanism for positive change and improvement, prioritizing quality over mere compliance with minimum acceptable standards.
- Cultivating a culture of trust, participation, and communication, where quality goals are reinforced through employee engagement.
Adopting a Quality Culture approach means the quality function needs to transition from traditional compliance-focused quality management to “quality beyond compliance.” This transition aims to integrate the quality function into the overall company business, enabling quality to become a competitive advantage and a strategic lever for business growth. Quality outcomes improve when more non-quality employees are involved in quality work (McKinsey, 2022).
One of the pioneers of the quality approach was Toyota Motor Co., Ltd., a company that had always placed a high value on customer satisfaction and quality. In 1949, Toyota introduced statistical quality control (SQC) and, in the following decades, won several prestigious awards for its commitment to quality, including the Deming Application Prize in 1965 and the Japan Quality Control Award in 1970 (Toyota, 2025). In the 1980s, a delegation of cross-industry business leaders from Europe visited a Toyota factory in Japan, eager to learn about the company’s famed Total Quality Management (TQM) approach. When they asked to see the organizational chart of the quality department, the Japanese hosts were initially confused and hesitant. After a while, they returned with the organizational chart of the entire factory, much to the surprise of the European delegation. “But where is the quality department?” the delegation asked. “Everyone is a part of the quality department,” the Japanese hosts replied, emphasizing the principle of “total participation” and the idea that everyone, from the factory floor to the executive suite, had a role to play in ensuring quality.
Fast forward to today, and the pharmaceutical industry has made significant strides in embracing this holistic approach to quality, driven by increased regulatory scrutiny, enhanced complexity of research, clinical development, and production processes, and other factors described above. In 2022, the FDA’s Center for Drug Evaluation and Research (CDER) moved to the next frontier to realize the vision for pharmaceutical quality today by promoting Quality Management Maturity (QMM) and stating that “Quality culture must be foundational for mature quality management.” (Center for Drug Evaluation and Research, 2022). This emphasis on QMM was sparked by alarming data: 62% of drug shortages between 2013 and 2017 were linked to manufacturing quality issues or quality defects in the finished product (Center for Drug Evaluation and Research, 2022). Drug shortages jeopardize patient care and severely harm a company’s reputation, highlighting the essential role of leadership support for a strong Quality Culture in ensuring business continuity.
 This emphasis on quality culture has permeated the industry, leading many pharmaceutical companies to sign a quality manifesto, a statement of an organization’s commitment to quality in all aspects of its operations. As Sanofi’s Quality Policy, signed by CEO Paul Hudson and Chief Quality Officer Maite Durrenbach, states: “Because our company is committed to a quality-driven culture, it is the responsibility of each and every employee to act in a compliant and ethical way, as we work together to transform the practice of medicine.” (Sanofi, 2024).
This emphasis on quality culture has permeated the industry, leading many pharmaceutical companies to sign a quality manifesto, a statement of an organization’s commitment to quality in all aspects of its operations. As Sanofi’s Quality Policy, signed by CEO Paul Hudson and Chief Quality Officer Maite Durrenbach, states: “Because our company is committed to a quality-driven culture, it is the responsibility of each and every employee to act in a compliant and ethical way, as we work together to transform the practice of medicine.” (Sanofi, 2024).
By prioritizing quality, CEOs take it on as their own responsibility, recognizing that quality is not just a business enabler, but a critical factor that directly impacts company performance,  manifested in increased revenue, market share, brand loyalty, higher levels of employee engagement and innovation, and — ultimately — better health outcomes for patients. Dr. Vasant (Vas) Narasimhan, CEO of Novartis, signed the current Novartis Quality Policy in 2023, stating among main principles: “Quality creates value. Our aim is to make quality a competitive advantage for Novartis, delivering high-quality, safe, and effective products on time, every time. This will make us more sustainable as a business and a better partner to our patients — now and in the years to come.” (Novartis, 2023).
manifested in increased revenue, market share, brand loyalty, higher levels of employee engagement and innovation, and — ultimately — better health outcomes for patients. Dr. Vasant (Vas) Narasimhan, CEO of Novartis, signed the current Novartis Quality Policy in 2023, stating among main principles: “Quality creates value. Our aim is to make quality a competitive advantage for Novartis, delivering high-quality, safe, and effective products on time, every time. This will make us more sustainable as a business and a better partner to our patients — now and in the years to come.” (Novartis, 2023).
Critically, quality policies must transcend mere words to become lived experiences. Pharma leaders need to regularly assess how employees understand and embody these policies, ensuring they do not become hollow slogans but rather drive meaningful cultural transformation.
Cultivating a quality mindset
To develop a Quality Culture, it is requisite to nurture great quality leaders who can drive and galvanize others. According to pharmaceutical quality veteran Dr. Anders Vinther and ex-FDA Commissioner Dr. Janet Woodcock, who developed a course on the subject, six traits differentiate great quality business leaders. To master these traits, quality leaders must adopt new ways of practicing quality that are proactive, go beyond compliance, and focus on the company, patients, and employees (Quality Business Administration, 2024).
Six traits of great quality business leader
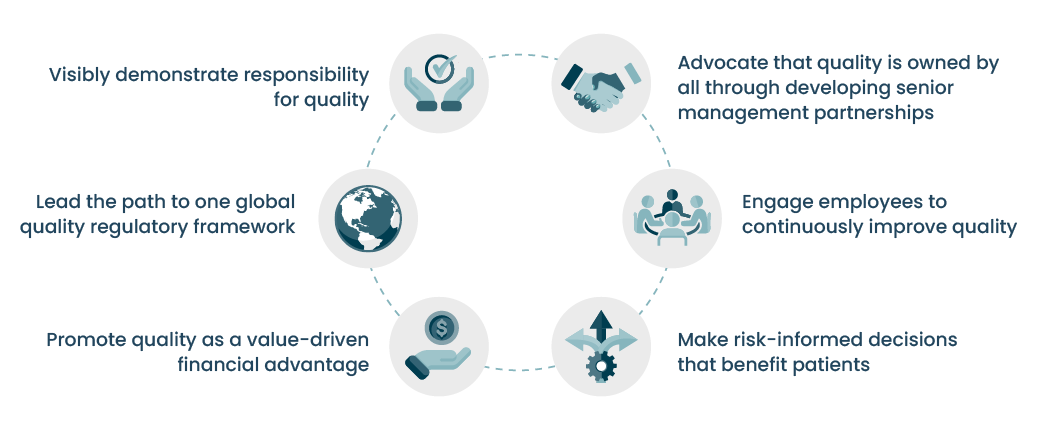
Quality Business Administration, 2024
Furthermore, to ingrain quality into the mindsets and behaviors of all employees and institutionalize Quality Culture at the enterprise level, it is critical to approach the process intentionally, holistically, and with a well-orchestrated plan. As with any cultural shift, it can be met with resistance, as employees may be hesitant or reluctant to change their established ways of working. It is essential to overcome this resistance and ensure that quality becomes ingrained in the organization’s DNA.
Here are some recommendations to consider.
Employee engagement. Developing a Quality Culture requires the active participation and engagement of all employees, from executive leaders to quality professionals and frontline employees. By fostering a quality mindset, employees can become more invested in the organization’s success and take ownership of quality initiatives. Providing adequate training, communication, and continuous education programs can empower employees and equip them with the necessary knowledge and skills to actively contribute to developing a Quality Culture.
Connecting quality to purpose. A strong Quality Culture, at its core, depends on employees understanding the deeper meaning of their work. Leaders must actively guide and listen to employees, helping them see how their daily quality decisions directly contribute to the company’s mission of saving and improving patients’ lives. This connection to purpose becomes particularly powerful when employees are empowered to lead continuous improvement initiatives, transforming quality from a requirement into a personal mission.
Investment. Implementing a Quality Culture delivers significant returns through reduced “costs of poor quality,” including fewer product destructions and market actions and improved right-first-time (RFT) rates. While developing a Quality Culture requires dedication of resources to technology, infrastructure, and training, the investment pays off through the prevention of costly quality issues. It is important to prepare a compelling business case for investing in a Quality Culture, including a demonstration of the potential cost savings, revenue growth, and competitive advantage that can be gained through a strong Quality Culture.
Reinforcement. Recognizing and appreciating employees who demonstrate quality-focused behaviors can help reinforce the importance of Quality Culture and motivate employees to continue to prioritize quality in their work. Providing regular feedback and recognition to employees fosters a culture of continuous improvement and encourages employees to take ownership of quality initiatives.
Success measurement. Establishing clear quality metrics and targets can help pharmaceutical companies measure progress towards achieving a Quality Culture and identify areas for improvement. Regularly reviewing and analyzing quality data can help pharmaceutical organizations identify trends and patterns, enabling them to take proactive measures to address potential quality issues before they become major problems. By taking a comprehensive and intentional approach to developing a Quality Culture, organizations can overcome resistance, empower employees, and reap the many benefits that a strong Quality Culture provides.
In conclusion, Quality Culture is a critical success factor for pharmaceutical companies. As Henry Ford once said, “Quality means doing it right when no one is looking.” By embracing Quality Culture, companies can create a unique selling point, strengthen patient and community relationships, and ensure patient safety, culminating in a longstanding, established culture of trust.








